FSMA 204: How produce-based food producers can prepare for changes
As produce-based food producers around the globe emerge from pandemic restrictions, they are encountering, in many cases, significant supply chain issues and a changing regulatory environment.
The Food and Drug Administration (FDA) is continuing to implement the Food Safety Modernization Act (FSMA); Section 204 will go into effect in 2023.
In January 2011, President Obama signed FSMA into law, and the FDA and food industries have been integrating the sweeping changes since. Now, food producers will need to address compliance with Section 204 by 2025. But despite the reforms that have already fundamentally changed food safety in the industry, compliance with 204 could mean some of the biggest changes yet.
So what do produce-based food producers need to do to prepare for the FSMA 204 rule change? The answer is: potentially quite a bit. But to understand what must be done, let’s review what changes FSMA 204 entails.
What is FSMA Rule 204?
In a nutshell, 204 requires producers to establish and maintain detailed records to facilitate traceability throughout the supply chain. In rule 204, the FDA expanded the lists of foods requiring recordkeeping. This enhanced tracking aims to promote a faster response to foodborne illness outbreaks and credible threat events. The changes coming with the implementation of 204 mean a fundamental shift from the current “one up, one back” approach.
Creating end-to-end traceability
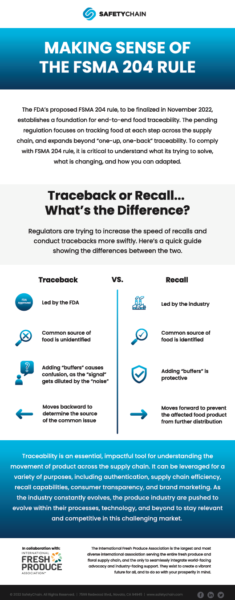
There’s no question that resiliency and visibility in the industry are necessary now more than ever. However, lack of preparation or haphazard implementation of traceability processes could end up costing more than some smaller operations are able to afford. For producers who have not yet begun integrating 204 requirements, there is still time — but the window is quickly closing.
The FDA can request that a business responds within 24 hours with Critical Tracking Events (CTEs). CTEs are simply records of events within the business process that are a part of ensuring end-to-end traceability. A CTE occurs when a portion of food is grown, received, transformed, created or shipped. And each CTE has its own requirements, called Key Data Elements (KDEs), for recordkeeping.
Each CTE must have a traceability lot code linked to the relevant KDEs. Traceability lot codes are usually alphanumeric descriptors assigned to a lot of food that has been created, originated, or transformed. These codes are intended to make it easier for electronic tracking in addition to visual tracking.
And producers must create and store all of this information in traceability program records. For producers still collecting data manually, this could open them to significant risk. With 204, the FDA plans to create an efficient, electronic end-to-end traceability record resulting in total transparency.
Digital transformation can facilitate FSMA 204 compliance
Producers — and indeed the food industry as a whole — have not all moved away from paper-based and manual recordkeeping systems. Some have implemented partial digitization, while others have adopted plant-wide automation and plant management software. However, viewing 204 as a hurdle to jump could be a short-sighted approach. There are significant opportunities to create more efficient processes that will positively impact already razor-thin margins and help make producers more competitive within their niches.
Additionally, integrating digital tools for recordkeeping and traceability programs will have global implications for producers. Compliance with 204 and the adoption or improvement of digital recordkeeping can make producers more resilient even in more volatile markets by allowing them to respond far more rapidly to supply chain disruption or market instability in a particular region.
RELATED: Traceability rule changes looming with FSMA 204
Finally, producers can benefit from more target and specific tracebacks and recalls with electronic access to end-to-end traceability. Rather than casting a wide net and destroying product in a “just in case” scenario, producers can know which specific lots are part of a foodborne illness investigation or a food supply threat. This precise targeting could result in cost savings and mitigate damage to a brand’s reputation.
FSMA 204 modernizes food safety through traceability
The integrity of the food supply is a vital aspect of public safety. FSMA 204 seeks to illuminate the often murky route that many foods take from origin to table. In today’s world, food may travel only a few miles or include elements that have moved through multiple countries. Actively working to implement compliance measures can give producers the tools to succeed in delivering safe food to customers.
— Tiffany Donica is a Customer Success Coach at SafetyChain Software. She has 18-plus years of experience in QMS systems integrations and continual process improvement projects relating to HACCP, Root Cause Analysis, and lean manufacturing. She is an expert in GFSI, BRC and SQF, and has experience in food manufacturing facilities as a: QA Manager at Epi Breads; Director of Quality and Continuous Improvement at Surlean Foods; Senior Manager, Food Safety & Quality Systems, at CTI Foods; and QA Manager at Five Star Custom Foods.







